Question #b8339
1 Answer
Jul 31, 2017
Explanation:
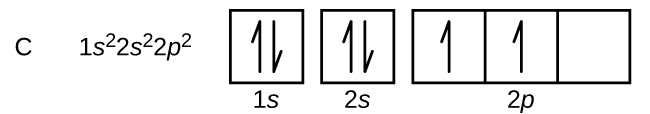
Assuming a carbon atom with a charge of 0:
The first two electrons always occupy the 1s energy level, as it is lowest in energy.
The next two will occupy the 2s energy level, as it is lower in energy than 2p.
The final two electrons will occupy different suborbitals, according to the Pauli Exclusion Principle, which essentially means "one in each before two in any."