Do electron clouds overlap when hybridization occurs?
1 Answer
First of all, electron clouds CAN overlap, and they must, to form chemical bonds in the first place!
The attraction of nucleus
And so, they must be able to overlap to form hybridized atomic orbitals in a linear combination.
Take the
#Psi_(sp^3) = c_1psi_(s) + c_2psi_(p_x) + c_3psi_(p_y) + c_4psi_(p_z)# where each wave function
#psi# represents a given pure atomic orbital, with weighted contributions given by#c_i# . As a result,#Psi_(sp^3)# represents the#sp^3# -hybridized orbital.
All that the above equation says is that hybridized orbitals form by the overlap of pure atomic orbitals.
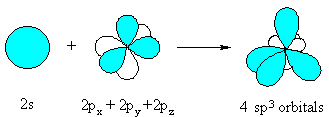
They achieve an intermediate energy between the original orbitals, so that all the hybridized orbitals are:
- the same energy
- the same look/symmetry
So, in
This then allows the orbitals to align themselves along the internuclear axes, and bond by head-on overlap (meaning,

