Which of these is the best Lewis acid?
#A)# #"BPh"_3#
#B)# #"BPh"_4^(-)#
#C)# #"B"("C"_6"F"_5)_3#
#D)# #"B"("C"_6"F"_5)_4^(-)#
1 Answer
Well, I would hope that
And in case you are not convinced, this paper briefly mentions that
These are all boron-class acids, i.e. they are derivatives of
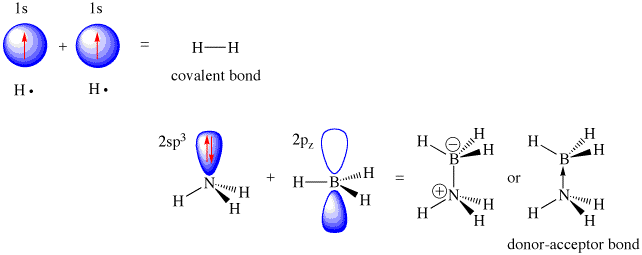
(the image depicts
#"NH"_3# acting as a Lewis base and#"BH"_3# acting as a Lewis acid.)
That empty
To your answer choices, these look like:
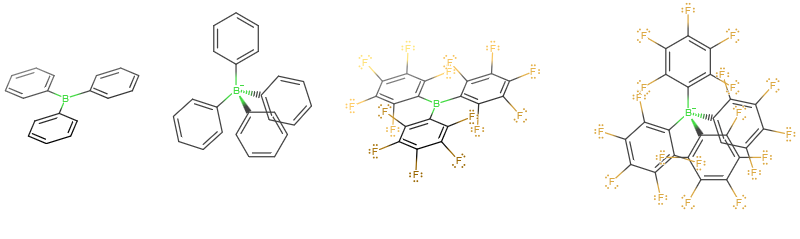
The four-coordinate, tetrahedral boron compounds (having the maximum possible number of bonds boron can make) are not Lewis acids, because there is no empty orbital on the central atom to accept electron density anymore. So, we can eliminate
However, in
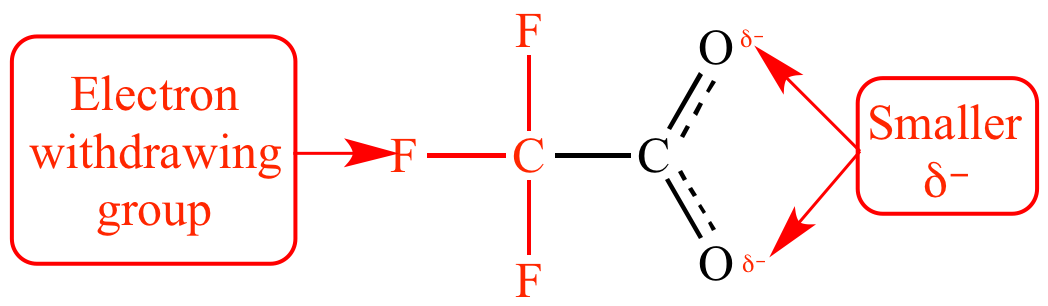
These inductively withdraw electron density from the central atom, so that boron becomes extremely electropositive. That greatly increases the tendency of the central

