How do we know where the bonding, antibonding, and nonbonding molecular orbitals are compared to the atomic orbitals the atoms start with? Do they always interact?
1 Answer
It always depends on the relative energy of a given atomic orbital with another atomic orbital, when it comes to where the antibonding molecular orbital (MO) lies.
I can present four general cases:
- Two atomic orbitals close to each other (homonuclear diatomics).
- Two atomic orbitals farther away from each other (heteronuclear diatomics).
- Two atomic orbitals too far away from each other (commonly occurring but not usually shown in textbooks to save space).
- Two atomic orbitals in range to interact with a third (often, though this can be difficult to understand).
For simplicity, we assume the orbitals in question have the correct "symmetry", interact only based on energy, and are sigma interactions. In reality it is a simplification.
CASE I: CLOSE ENOUGH IN ENERGY
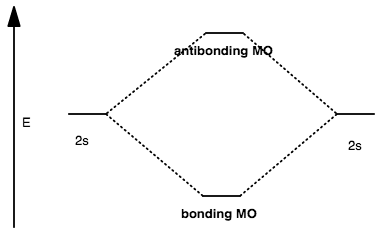
The antibonding MO is always higher in energy than the starting atomic orbitals.
CASE II: LARGE DIFFERENCE IN ENERGY
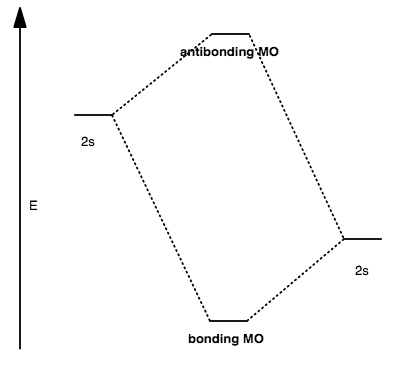
The antibonding MO is always higher in energy than the higher-energy atomic orbital.
The orbital is said to be more centered on the atom whose atomic orbital is closest in energy to it. If the antibonding MO contains electrons, the electrons then primarily belong to that atom.
CASE III: TOO LARGE DIFFERENCE IN ENERGY (NO OTHER ORBITALS IN RANGE)
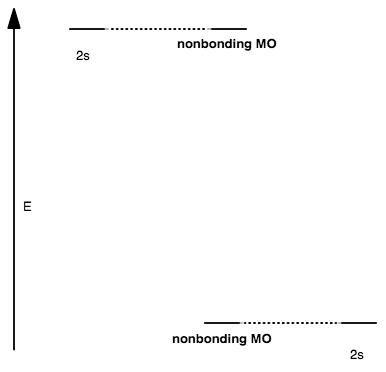
When the orbitals are too far apart in energy, AND if no other orbitals are in range to interact, then the orbitals are nonbonding and no antibonding MO exists.
An example of this is in
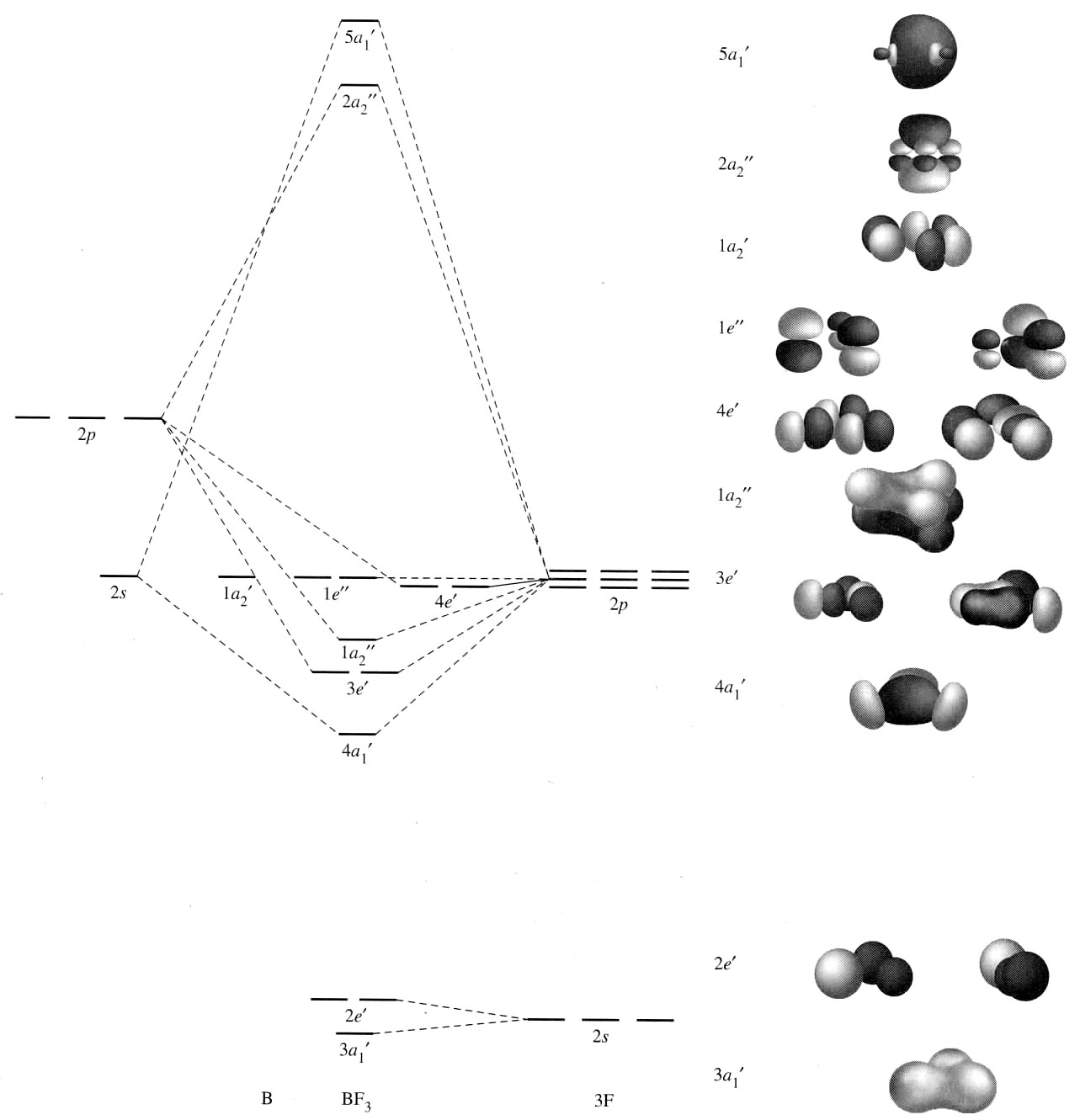
CASE IV: INTERACTIONS WITH A DIFFERENT ORBITAL
Sometimes, more than two orbitals can interact at once. In this case, you still spit out the same number of MOs as the number of atomic orbitals you started with.
However, you would have one mostly-nonbonding MO amongst them. In this case, the orbital previously conceived to be antibonding is now nonbonding and we generate a new antibonding MO.
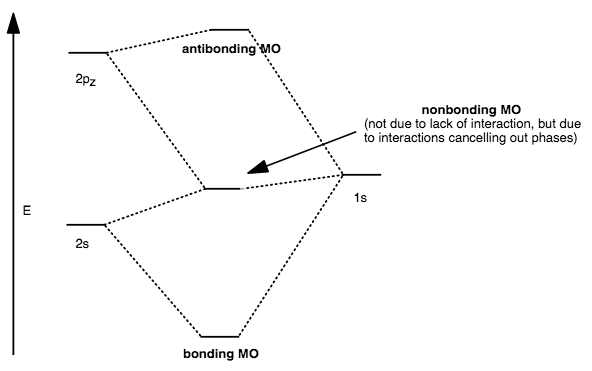
And this actually happens a lot...
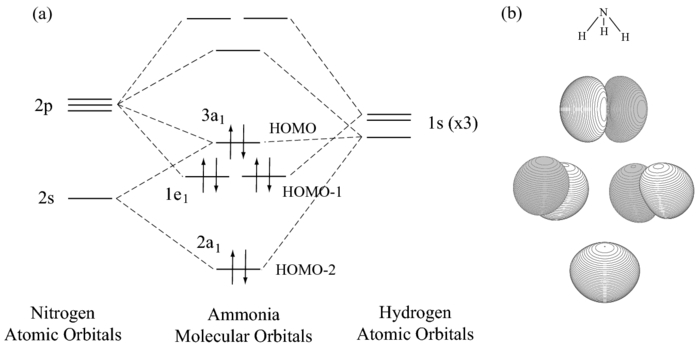
Here,
- the
#4a_1# (unlabeled, above the#3a_1# ) would be the antibonding MO. - the
#3a_1# holds the lone pair of electrons on#"NH"_3# , but it has some bonding character since it is lower in energy than the right-hand#1s# group orbitals of the#3 xx "H"# atoms. - the
#2a_1# would be the bonding MO.

