Question #9267e
1 Answer
The 3d orbitals will fill up first.
Explanation:
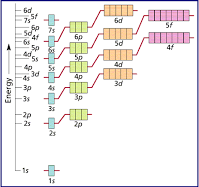
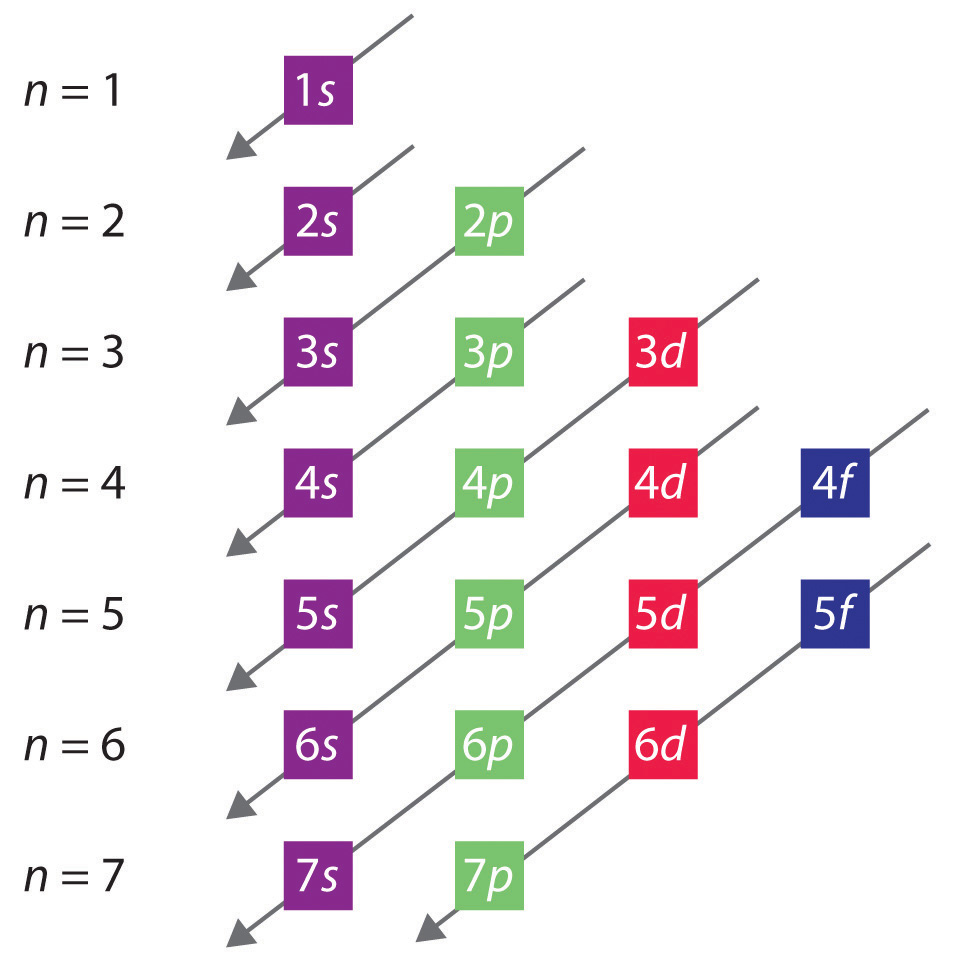
These are charts* to help you further, but basically, the energy of the 4p orbitals is slightly higher than the energy of the 3d orbitals and therefore, the 3d orbitals are filled first.
*the second chart can be used by starting at the top and following the arrow from left to right to see the order of the electron configuration. (1s 2s 2p 3s 3p....)


