An element with the general electron configuration for its outermost electrons of #ns^2np^1# would be in which element group?
1 Answer
Group 13.
Explanation:
As you know, the periodic table is organized in blocks that correspond to the location of an element's highest-energy electrons.
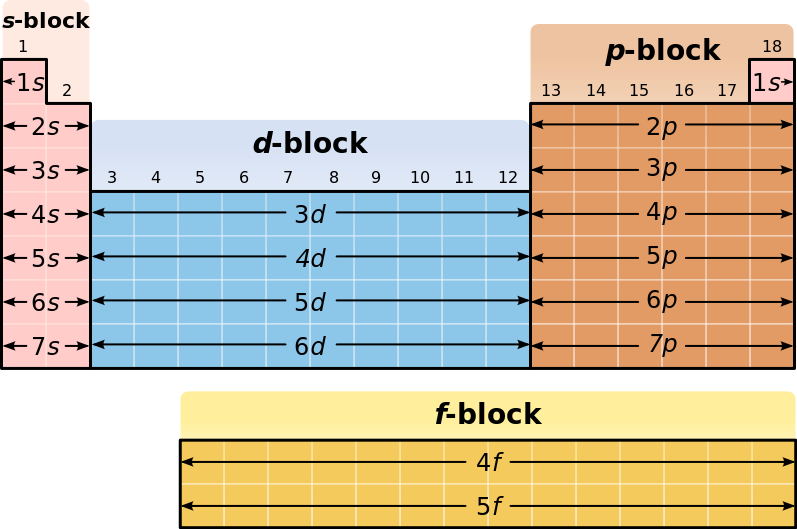
Elements that have their highest-energy electrons in the
In your case, the electron configuration of the outermost electrons, i.e. valence electrons, in an atom looks like this
#n s^2 np^1#
Now, the highest-energy electron is located in a
This places the element in the
For any energy level, i.e. period in the periodic table, the first element located the
The electron configuration for the outermost electrons of an element located in group 13 will thus be
#ns^2 np^1#
Since this matches the electron configuration given to you, you can say that this unknown element is located in group 13.
Similarly, you will have
#ns^2 np^2 -># an element located in group 14#ns^2 np^3 -># an element located in group 15#ns^2 np^4 -># an element located in group 16#ns^2 np^5 -># an element located in group 17#ns^2 np^6 -># an element located in group 18

