Can the octet rule be broken?
1 Answer
May 15, 2018
But of course...
and we see that in many molecules, like the trigonal bipyramidal
Here,
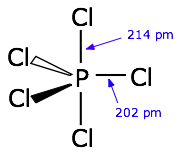
Here,
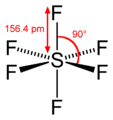
Here,
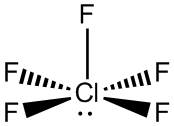
This is possible because all three central atoms have access to orbitals on the third quantum level,

