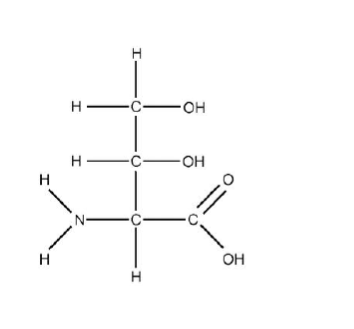
Circle all chiral centers in the molecule shown here?
1 Answer
Dec 1, 2016
Carbons 2 and 3 are chiral centres.
Explanation:
A chiral centre must be attached to 4 different groups.
Let's examine each carbon atom in your molecule: 2-amino-3,4-dihydroxybutanoic acid.
Carbon 1
Carbon 1 is not a chiral centre because it has only 3 groups attached.
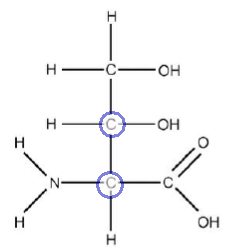
Carbon 2
Carbon 2 is a chiral centre because it has 4 different groups attached.
They are
Carbon 3
Carbon 3 is a chiral centre because it has 4 different groups attached.
They are
Carbon 4
Carbon 4 is not a chiral centre because it has two identical groups (

