Does resonance help radical stability?
1 Answer
Mar 1, 2017
Absolutely!
Explanation:
Recall that radical stability follows the trend:
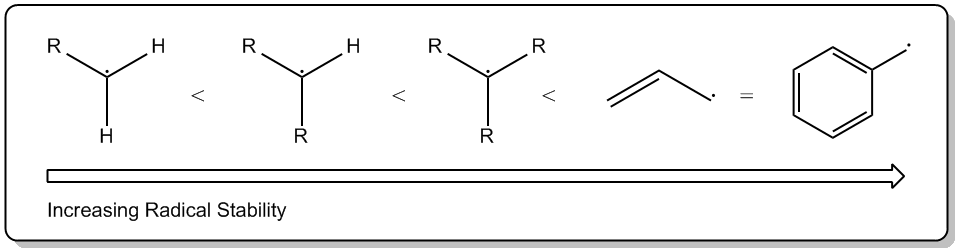
From left to right we have a primary (
Radicals have an
Consider this allylic radical
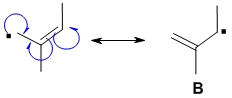
Using half arrows to denote electron movement, resonance of an allylic radical can be shown. Recall that resonance shows how charges can be delocalized among multiple atoms.
The same can be seen for a benzylic radical
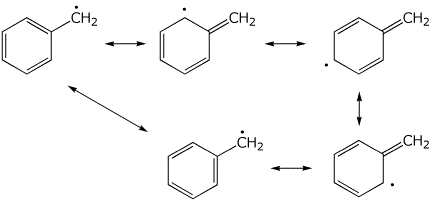
Remember: The more resonance forms a structure has, the more stable it is
