Draw the canonical forms for #CH_2=CH-CH=CH-CHO#, and compare their relative stabilities?
2 Answers
See explanation.
Explanation:
If we consider the delocalization through the entire backbone of the molecule, the canonical forms (resonance structures) could be represented as follow:

The structure with no charges would be the major contributor and therefore the more stable form. The carbocation would be very unstable especially on a primary carbon atom
See the discussion below.
Explanation:
STRUCTURES
The main thing to remember in drawing resonance structures is that electrons move towards a positive charge or, failing that, to the most electronegative atom.
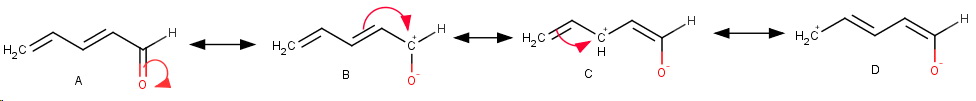
The starting ketone A has no positive charge, so we start by moving the carbonyl π electrons onto the electronegative
Next, we move the adjacent π electrons to the newly-created carbocation to get C.
Finally, we repeat the process to get D.
STABILITIES
I predict the order of stabilities to be
A > B > C > D
A is the most stable contributor, because it has no separation of charge.
B, C, and D are all higher energy contributors because they have separation of charge (it takes energy to move a negative charge away from a positive charge).
They have about the same energy, but I predict B to be the most stable of the three, because it has less charge separation.
Next comes C.
Finally, D is the least stable because (a) it has the greatest charge separation and (b) because it is a primary cation.
Note that in B, C, and D the negative charge stays on the electronegative oxygen atom.


