Draw the Lewis structure for 1,1-dimethylhydrazine #[(CH_3)_2N NH_2]#, a compound used as a rocket fuell. What is the hybridization for the two nitrogen atoms in this molecule? What orbitals overlap to form the bond between the nitrogen atoms?
1 Answer
Jul 20, 2018
Are not the nitrogen atoms
Explanation:
The bonding in the hydrazine is electron precise and each nitrogen atom is
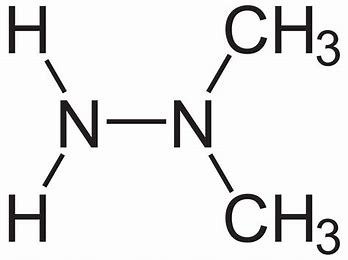
You will have to add in the lone pairs...

