Explain Gay Lussac's law by means of a particle diagram?
1 Answer
Gay-Lussac's Law of Combining Volumes states that when gases combine in chemical reactions, their volumes are always in the ratio of small whole numbers.
Explanation:
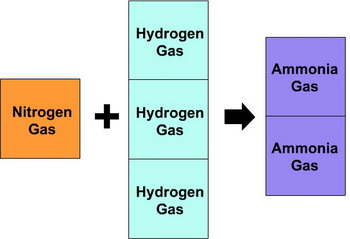
Gay-Lussac found that 1 volume of nitrogen and 3 volumes of hydrogen always reacted to form 2 volumes of ammonia.
(from www.molechemistry.info)
The volume ratios were always nitrogen:hydrogen:ammonia = 1:3:2.
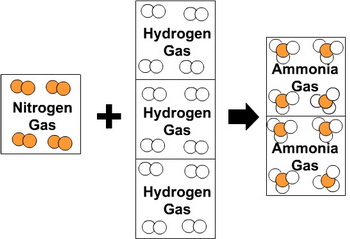
Based on Gay-Lussac's results, Amedeo Avogadro theorized that equal volumes of gases at the same temperature and pressure contained equal numbers of molecules (Avogadro's law).
This meant that Gay-Lussac's results could be expressed as
If this were true, the results made sense only if nitrogen and hydrogen were diatomic molecules and ammonia had the formula
(from molechemistry.info)
Suddenly, chemical reactions became understandable.
Chemists could write the reaction as
It all made sense!

