For example, i am given a problem, how do ik if it' a diluton problem. I know that for dilution the concentration decreases and the volume increases?
1 Answer
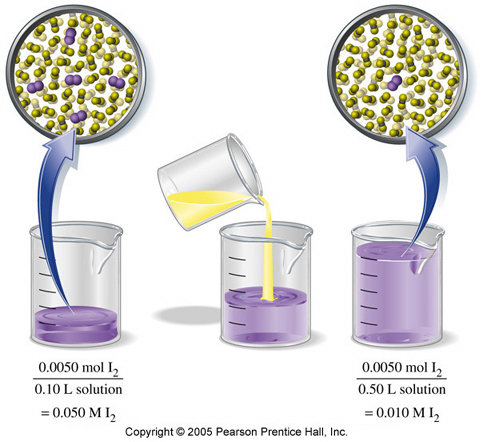
The concentration decreases because the volume increases, if the number of moles is kept constant.
That's the key point to keep in mind when dealing with dilutions - the number of moles of solute remains unchanged.
When you dilute a solution, you essentially increase its volume while keeping the same amount of solute present. Since molarity is defined as moles per liters of solution, an increase in volume will mean a decrease in concentration.
The equation to use for dilution calculations is
A dilution problem will usually imply that you start with a solution that has a certain concentration and volume, and either add more solvent to that solution, or take a sample from it and add solvent to the sample.
Whatever the case may be, try to focus on moles of solute and keep an eye on how the volume changes.

