How can you determine the electron configuration without a periodic table?
1 Answer
For that we have to use the aufbau principle
Explanation:
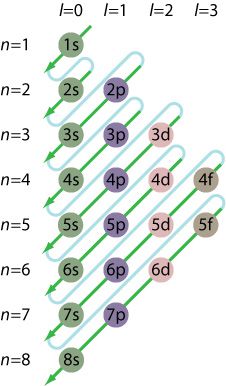
We have to start from 1s and gradually move with the arrows indicated in the diagram above.
For example, writing the electronic configuration of Fe(28)
we move according to the diagram i.e 1s then 2s then 2p then 3s and so on.
I recommend that you memorize the aufbau principle. Once you memorize it, you don't need anything else except the atomic number of the element to write its electronic configuration.


