At its core, the meaning of #sp^n# is that one #s# orbital mixes with #n# number of #p# orbitals close in energy to form degenerate (same-energy) hybridized atomic orbitals that can allow access to more electrons than available from "pure" (#s#, #p#, etc) atomic orbitals for bonding.
-
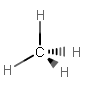
#sp^3# bonding involves using four #sp^3#-hybridized atomic orbitals, so it must have four electron groups. EX: #"CH"_4#
-
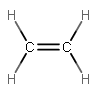
#sp^2# bonding involves using three #sp^2#-hybridized atomic orbitals, so it must have three electron groups. EX: #"BH"_3#, #"H"_2"C"="CH"_2#
-
#sp# bonding involves using two #sp#-hybridized atomic orbitals, so it must have two electron groups. EX: #"H"-"C"-="C"-"H"#, #:"C"-="O":#
I've explained #sp^3# and #sp^2# hybridization below, and from that, I think you can imply what #sp# hybridization is.
#\mathbf(sp^2)#-HYBRIDIZED BONDING
For instance, #"H"_2"C"="CH"_2# involves two #sigma# bonds (one for each single bond), and then one #sigma# and one #pi# bond (used in one double bond), so three electron groups are needed, but 4 electrons need to be donated by carbon.
Since carbon has 4 valence electrons, but its #p# orbitals (which are highest in energy) only contain 2, it needs to mix two of the three #2p# orbitals with the #2s# orbital to make use of 2 more valence electrons. This is favorable because it involves the lowering of the energies for two of the #2p# orbitals, increasing stability.
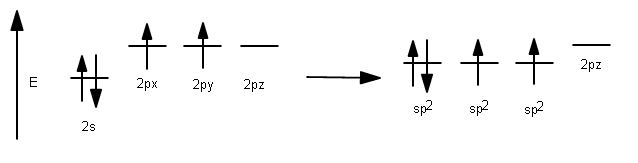
This results in the usage of three #sp^2# hybrid orbitals to bond: the ones with one electron for #sigma# bonding to hydrogen, and the one with two electrons for incorporating one #sigma# and one #pi# bond with the other carbon.
1 #2s# orbital had been incorporated, and 2 #2p# orbitals had been incorporated, so it is called #sp^2#, having #33%# #s# character and #66%# #p# character.
#\mathbf(sp^3)#-HYBRIDIZED BONDING
A similar reasoning follows for #sp^3# bonding. Let's take #"CH"_4# as an example. It needs four electron groups, and it needs to make four IDENTICAL #sigma# bonds (one for each single bond).
4 valence electrons are needed from carbon, but only 1 electron needs to be contributed per #sigma# bond. So, we need four separate degenerate hybrid orbitals to make each #sigma# bond. Therefore, all three #2p# orbitals must mix with the #2s# orbital and stabilize in energy overall to get four degenerate hybrid orbitals.
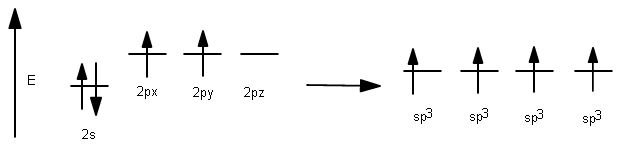
This results in the usage of four #sp^3# hybrid orbitals to bond: the ones with one electron allow #sigma# bonding to hydrogen.
1 #2s# orbital had been incorporated, and 3 #2p# orbitals had been incorporated, so it is called #sp^3#, having #25%# #s# character and #75%# #p# character.
I think from here, you can imply what #sp# hybridization means. (Hint: It can also be called #sp^1# hybridization.)

