How can you test for ketones?
1 Answer
Ketones and aldehydes react with 2,4-dinitrophenylhydrazize. A positive test gives a yellow or orange precipitate.
Explanation:
Ketones and aldehydes react with a reagent called 2,4-dinitrophenylhydrazine (2,4-DNPH) to form a yellow or orange precipitate within a few minutes. Although this reaction has limitations, it is still a good test for aldehydes and ketones.
2,4-DNPH is provided in the lab as a solution. A solution of 2,4-dinitrophenylhydrazine in a mixture of methanol and sulphuric acid is known as Brady's reagent.
The flowing examples show the reaction of 2,4-DNPH with propanone and ethanal.
The reaction with propanone (a ketone) is shown below.
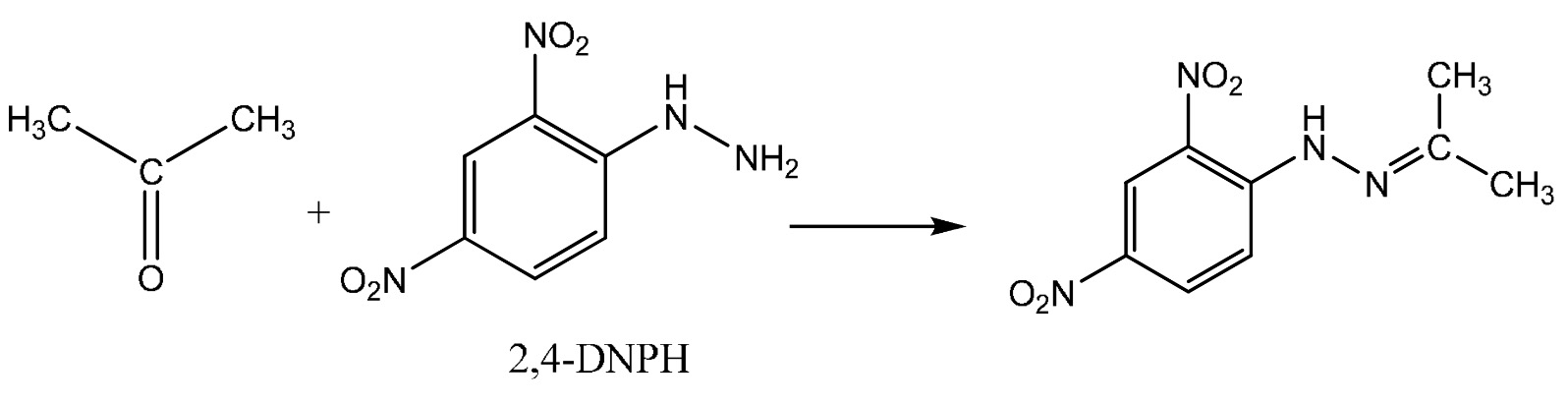
The product of the reaction with propanone is called propanone 2,4-dinitrophenylhydrazone
The reaction with ethanal (an aldehyde) is shown below.
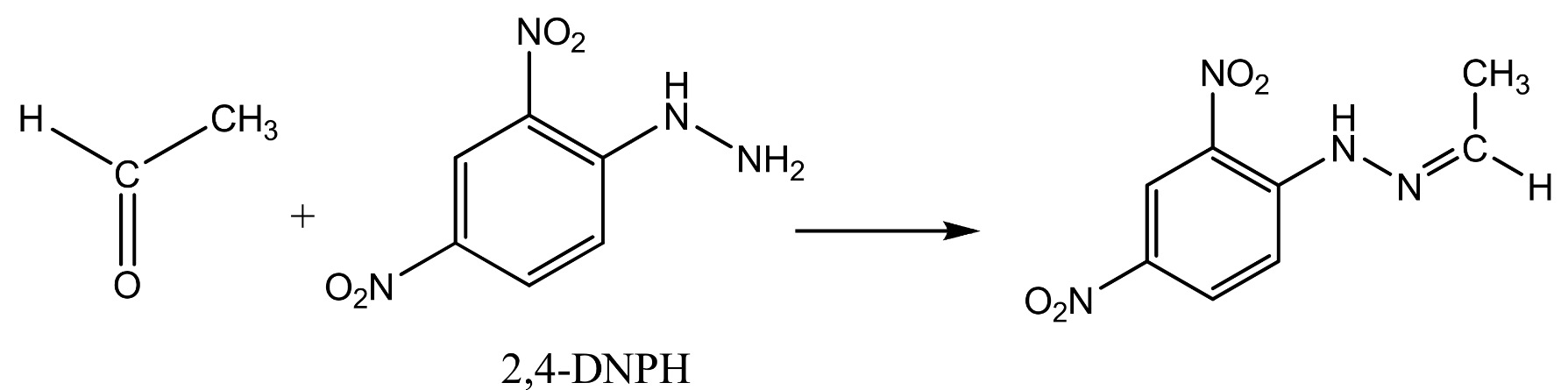
The product from the reaction with ethanal Is called ethanal 2,4-dinitrophenylhydrazone;
The above reactions are known as condensation reactions. A condensation reaction is one in which two molecules join together with the loss of a small molecule in the process. In this case, that small molecule is water.
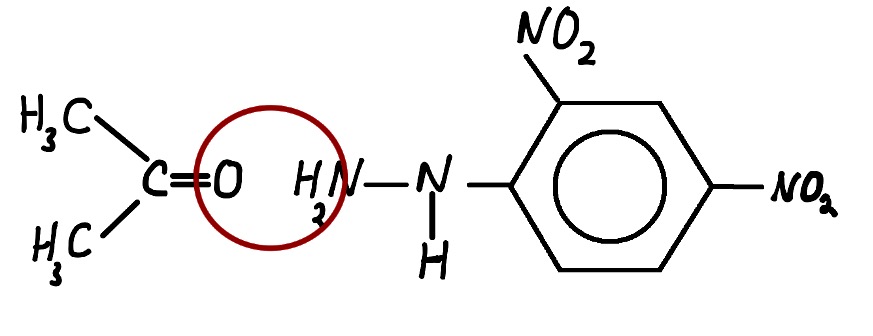
Note that water is a byproduct of the reaction which is often omitted from the equation.
