How do aldehyde and ketone structures differ?
1 Answer
Mar 3, 2016
They both have a oxygen atom double-bonded to a carbon.
Explanation:
The difference is that in a ketone (suffix -one) the
This combination of
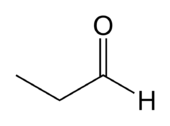 propanal
propanal
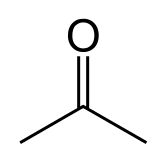 propanone
propanone
(pictures from wikipedia)

