How do metal halides work?
1 Answer
Maybe this is referring to compounds like
-
Generally, since the more electronegative halogens (
"Cl" and"Br" ) are more electronegative than the metal, the metal center becomes Lewis acidic. -
Furthermore, the halogens own a significant portion of the molecular orbitals, because their orbitals are far away in energy from the metal's orbitals. This allows a fourth halide on a negatively-charged metal halide to act as a nucleophile.
All of this is to the extent that we can expect normally strange things to occur.
FRIEDEL-CRAFTS ACYLATION
For example,
Although, formation of the acylium ion is the slow step (the rate-determining step), so it's still difficult like we expect it to be.
FRIEDEL-CRAFTS ALKYLATION
A similar process occurs in the Friedel-Crafts Alkylation, which can use
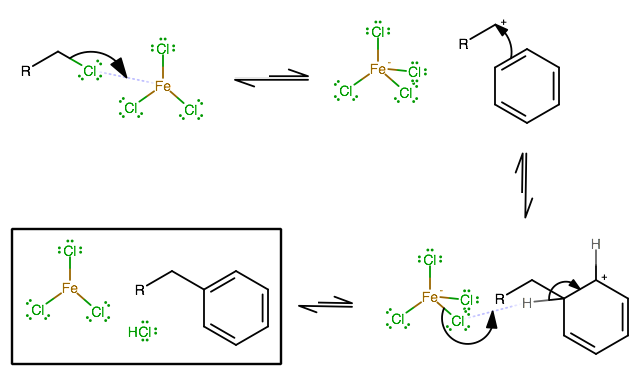
In this case, you form the normally-unstable primary carbocation, which clearly should be the slow step, as we almost never see a halide leaving group depart a primary alkyl halide and leave behind a primary carbocation.
You should see the similarities in metal halide behavior between these two reactions:
- The metal center is Lewis acidic, convincing normally unstable cations to occur more easily.
- The halogen owns a significant portion of the molecular orbitals, so the fourth halide can act as a nucleophile and come straight off a four-halogen metal halide.

