How do you calculate the dipole moment of water?
1 Answer
Let's suppose we put water on the xy-plane like so:
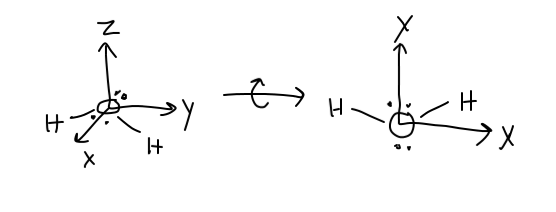
The dipole moment is calculated by looking up the dipole moment contributions from each
The net dipole points through oxygen down the y-axis in the negative direction.
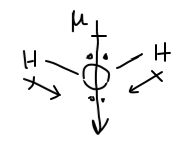
Note that the dipole projection along the x directions cancel each other out; if the left dipole contribution was pointing to +x, the right contribution points to -x.
As a result, to calculate the net dipole, determine the projection of each dipole in the y direction, and then double it, since both
The
Take the angle used in the projection to be from the vertical until each
With that, and the fact that
#mu_"y,left contribution" = mu_("OH")xxcos(52.2388^@)#
#= "1.5 D" xx 0.612 = color(green)(0.9187)#
#mu_"y,right contribution" = mu_("OH")xxcos(-52.2388^@)#
#= "1.5 D" xx 0.612 = color(green)(0.9187)#
Finally, we sum them up because they are both in the same y direction:
#color(blue)(mu_"tot") = mu_"y,left contribution" + mu_"y,right contribution"#
#= 0.9187 + 0.9187 = color(blue)("1.837 D")# which is pretty close to the actual
#"1.85 D"# .
Likely the error was either from the referenced

