How do you count pi electrons in aromatic compounds?
1 Answer
- Since any one chemical bond (meaning only one line in bond line notation) contains at most two electrons, you can count two
#pi# electrons per double bond, and ignore the#sigma# electrons. - If you see lone pairs, consider the molecular geometry, and only the
#pi# electrons that are in the ring count towards aromaticity.
Here are some examples of rings that may or may not be aromatic:
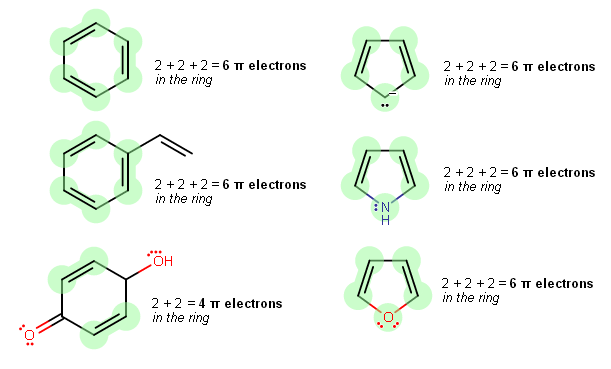
Note that the only
Counting from top to bottom, column-wise:
-
Aromatic, because
#4n + 2 = 6# #pi# electrons in the ring (with#n = 1# ), planar, fully conjugated all around, and cyclic. -
Aromatic, because
#4n + 2 = 6# #pi# electrons in the ring (with#n = 1# ), planar, fully conjugated all around, and cyclic. The#pi# electrons in the double bond outside of the ring do not count towards the#pi# electrons one considers for aromaticity. -
Nonaromatic, because
#4n + 2 ne 4# #pi# electrons, where#n# must be an integer. It's also not conjugated all around, so it's not antiaromatic. The#pi# electrons in the double bond outside of the ring do not count towards the#pi# electrons one considers for aromaticity. -
Aromatic, because
#4n + 2 = 6# #pi# electrons in the ring (with#n = 1# ), planar, fully conjugated all around, and cyclic. The lone pair is actually in a pure#2p# orbital perpendicular to the ring. Don't be fooled, as the alkyl carbon has an implicit hydrogen. -
Aromatic, because
#4n + 2 = 6# #pi# electrons in the ring (with#n = 1# ), planar, fully conjugated all around, and cyclic. The lone pair is actually in a pure#2p# orbital perpendicular to the ring, which means they count as#pi# electrons. -
Aromatic, because
#4n + 2 = 6# #pi# electrons in the ring (with#n = 1# ), planar, fully conjugated all around, and cyclic. Only one of the lone pairs is actually in a pure#2p# orbital perpendicular to the ring, which means those count as#pi# electrons. The other lone pair is actually in a#sigma# (actually,#sp^2# ) orbital, so it doesn't count. Thus furan is not antiaromatic.

