How do you count pi electrons in aromatic rings?
1 Answer
How many double bonds do you see?
Explanation:
Well, if it is a phenyl ring, you usually see 3 double bonds:
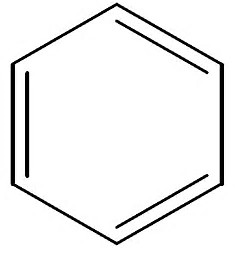
And thus 3 olefinic bonds, hence
This is more what you tend to see at undergraduate level. When we see this we UNDERSTAND that the
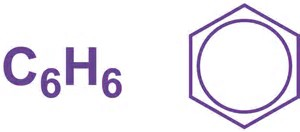
Note that both are EQUIVALENT representations. The formulaic representations are of common, real chemicals with distinct chemistry, for whose pattern we try to account. While we know that the former IS NOT
Note that there are other common aromatic systems, most notably the aromatic heterocycles, pyrrole, furan, and thiophene.

