How do you determine if a compound is meso when trans or cis is not listed? Such as in the example of 2,3-Pentanediol
1 Answer
You draw stereochemical formulas of the isomers and look for superimposable mirror images.
Explanation:
A molecule must have restricted rotation — a ring or a double bond — in order to have cis and trans isomers.
Pentane-2,3-diol has neither a ring nor a double bond, so it has no cis or trans isomers.
Here are the Fischer projections of its optical isomers.
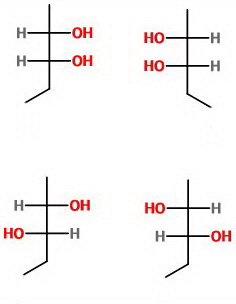
None of these isomers is a superimposable mirror image of the other, so there are no meso compounds.
Cyclopentane-1,2-diol does have restricted rotation, so it has cis and trans isomers.
The stereoisomers of trans-cyclopentane-1,2-diol are
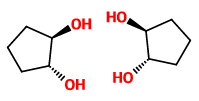
The mirror images are not superimposable, so these structures are enantiomers.
.
The stereoisomers of cis-cyclopentane-1,2-diol are
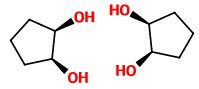
These two mirror structures are superimposable, so the cis diol is a meso compound.

