How do you draw a Newman projection for hexane while sighting down #C_2-C_3#?
1 Answer
You follow a series of steps.
Explanation:
Step 1. Draw the structure of hexane.
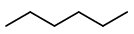
Step 2. Convert it to a wedge-dash structure at
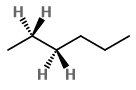
Step 3. Identify the groups on
The main chain of hexane is the horizontal zig-zag line of carbon atoms.
The groups on
Those on
Step 4. Draw a template for a Newman projection.
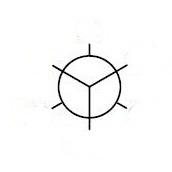
Step 5. Attach the groups to the carbons of your template.
View the molecule from the upper left.
The groups on
The groups on
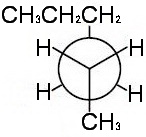
This is the most stable conformer, because it has the bulky methyl and propyl groups anti to each other.

