How do you make aldehyde from carboxylic acid?
1 Answer
Notice how a carboxylic acid, with a
That means you'll have to somehow reduce a carboxylic acid one step to get to an aldehyde.
Two common ways to reduce a carbonyl compound is with
#stackrel("NaBH"_4 " is suitable")overbrace("Acyl Halide" > "Anhydride" > "Aldehyde" > "Ketone") > stackrel("LiAlH"_4 " is suitable")overbrace("Ester" ~~ "Carboxylic Acid" > "Amide" > "Carboxylate Ion")#
Note that It is difficult to reduce a carboxylic acid to an aldehyde without accidentally reducing it further into an alcohol because
There are other ways to make a carboxylic acid more reactive, which will open up more pathways we can use, and one of them is to turn it into an acyl chloride.
So, we will have to do this in two or more steps. Two ways I can think of:
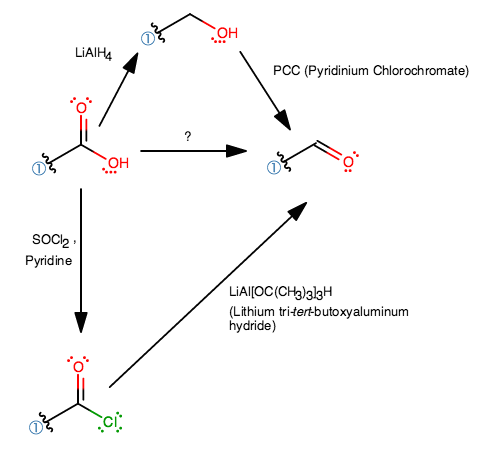
Like I said earlier,
Alternatively, you can turn the carboxylic acid into an acyl chloride using

