How do you measure ionic radii?
1 Answer
Jun 26, 2017
Usually the unit cell dimensions of ionic solids are interrogated.......
Explanation:
To a first approximation, ionic solids are considered to be close-packed spherical cations, and anions.......And ionic radii can thus be determined from structural data.
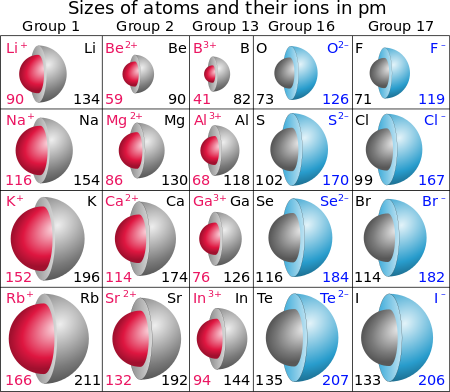
The data here are quoted in
Given the data in the diagram, why should ANIONS be larger than same Period CATIONS? Certainly if we interrogated the atomic radii, those atoms to the right of the Period as we face it are SMALLER than the atoms to the left.

