How do you predict the hybridisation of an atom in a molecule?
For example, the #N# atom in Pyridine is #sp^2# hybridised. But how do you find that?
For example, the
1 Answer
You look at the number of electron groups. There's more to it than that, but at a basic level...
The number of electron groups equals the number of orbitals used to construct the hybridized orbitals.
You might find it useful to look over this answer as well.
DISCLAIMER: Long answer!
PYRIDINE HYBRIDIZATION
In pyridine, what you have on the
- one connected to a carbon via a single bond
- one connected to a carbon via a double bond
- one that is a lone pair of electrons
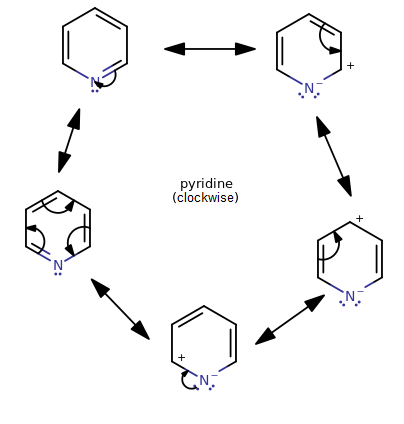
With three electron groups, you have the
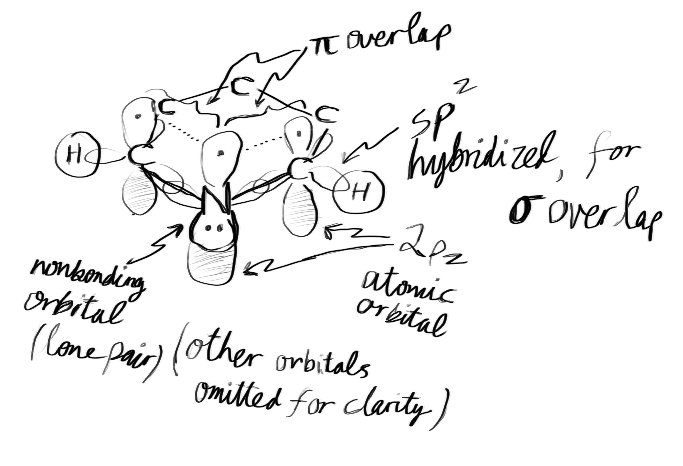
ORBITAL PERSPECTIVE OF PYRIDINE
Actually, the
- two hybridized
#sp^2# atomic orbital lobes partially occupied with one electron (both used to#sigma# bond). - one unhybridized
#2p_z# atomic orbital lobe partially occupied with one electron (used to#pi# bond within the ring), perpendicular to the ring. This is not included in the counting of electron groups, because it isn't hybridized. - one hybridized nonbonding
#sp^2# atomic orbital lobe (the third one of three) that holds the lone pair, parallel to the ring.
http://wps.prenhall.com/
Let's fill in the missing
The
The
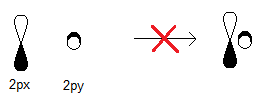
So, they, along with the
The hybridization must occur to make diagonal bonds that are all identical in energy and look.
The

