How does a carbon atom form lipids, carbohydrates, nucleic acids, and proteins?
1 Answer
Carbon has four valence electrons in its outermost shell and needs eight to become stable. Therefore, carbon can easily form covalent bonds with other atoms to form organic macromolecules.
Explanation:
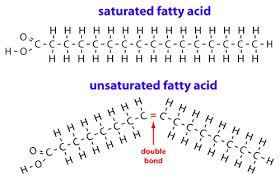
In the case of lipids, one carbon atom can form single covalent bonds with other carbon and hydrogen atoms to form saturated fatty acids. Sometimes carbon atoms will form double covalent bonds, and therefore form unsaturated fatty acids.
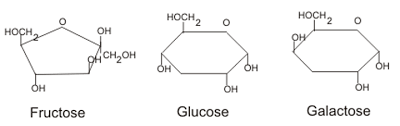
In the case of carbohydrates, five or six carbon atoms will combine with hydrogen and oxygen to form monosaccharides. These monosaccharides may then link together via glycosidic bonds and form polysaccharides.
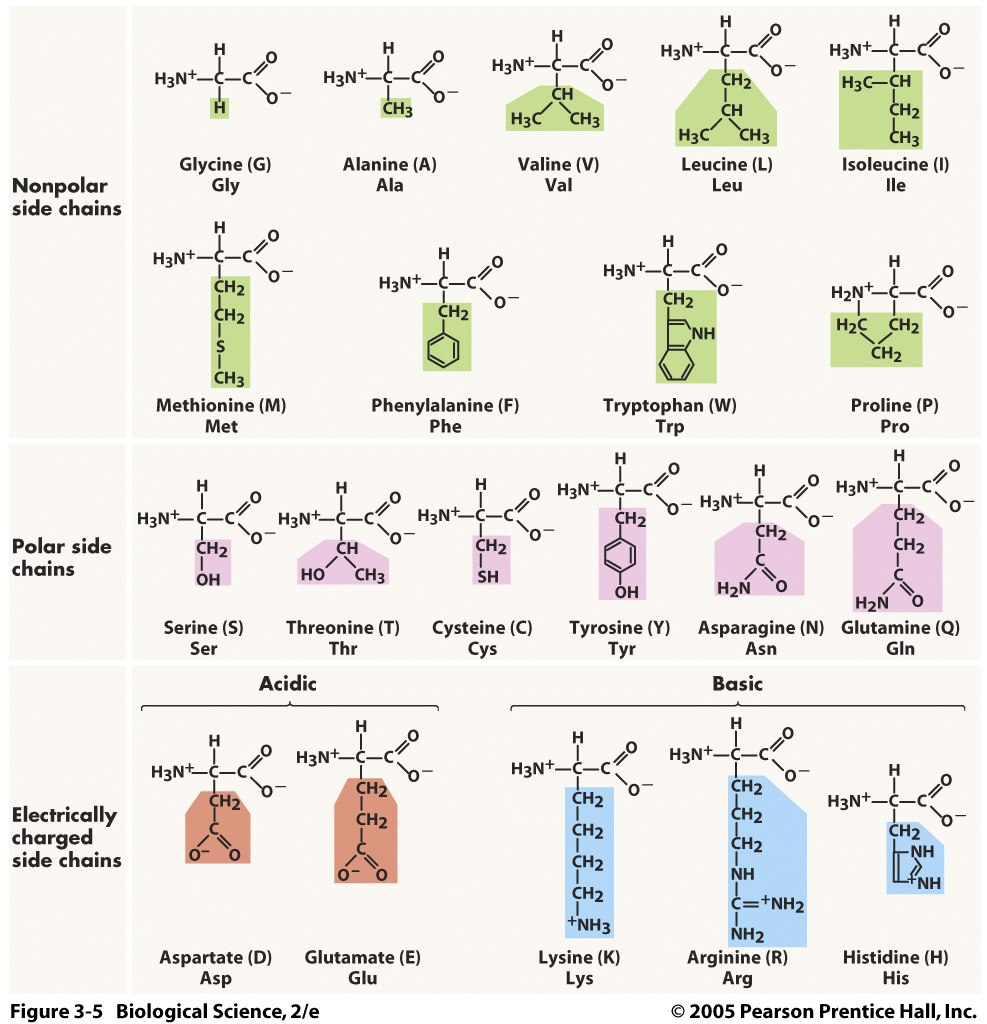
In the case of proteins, carbon forms the backbone of amino acids that contain an amino group (NH2), a carboxylic acid (-COOH), and a side chain specific to each amino acid.
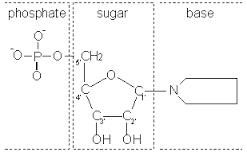
Nucleic acids are very large molecules consisting of chains of nucleotides. These nucleotides consist of a five-carbon sugar, a nitrogenous base, and a phosphate group.