How does a hydrolysis reaction separate a polymer into its monomers?
1 Answer
By re-introducing water, breaking the covalent bond, and re-attaching the
Explanation:
This works only for condensation polymers, in which molecules join together — losing small molecules like water as by-products.
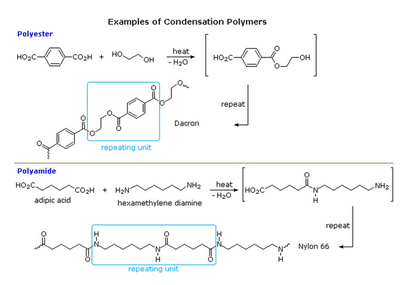
(from www.boundless.com)
The process of splitting the bond between the monomers is called hydrolysis.
Hydrolysis means “to break with water”.
Since a water molecule was lost during the condensation, hydrolysis brings the water back.

To separate the monomers, the functional groups
We can view it as if the covalent bond is broken between the atoms holding the monomers to each other, and then a water molecule is introduced.
The water molecule splits into an
Hydrolysis is an energy releasing process. Living organisms harvest energy through hydrolysis of chemical bonds.


