How does charge affect a lewis structure?
1 Answer
Well, a Lewis structure should reflect our idea of formal charge....
Explanation:
And let us take nitrite ion as an exemplar.....we gots
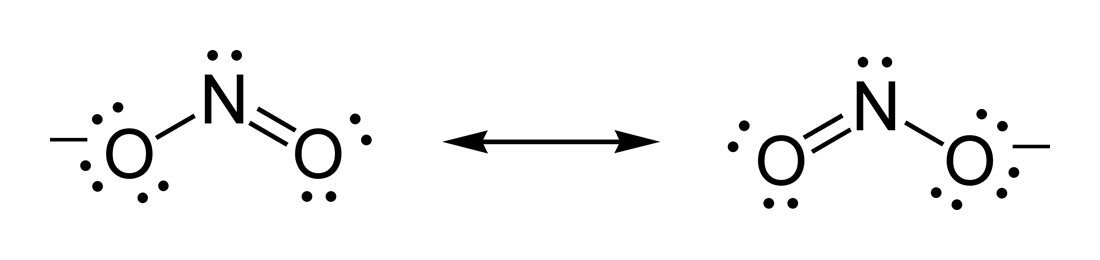
And either resonance isomer does distribute the nine electron pairs...
Nitrite ion has a formal negative charge.....given the diagram, the left hand structure has a oxygen atom with with 7 valence electrons, i.e. with the two inner core electrons, there are 9 electrons associated with this atom, and thus a formal negative charge, i.e. for oxygen
On the other hand, the central nitrogen formally owns 5 valence electrons, and with the two inner core electrons, has 7 electrons to balance the 7 nuclear charges....and thus nitrogen is represented as a neutral species.

