The higher the temperature, the faster a non-biological reaction tends to occur. For #S_N1# and #S_N2# reactions, the higher the temperature, the more elimination products you get. The more elimination products you get, since the amount of reactant is limited, the less substitution products you get, as well.
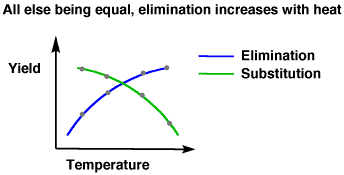
This is because the activation energy for a particular reaction with particular reactants is higher for elimination reactions than for substitution reactions of those same reactants.
Why is that? Consider molecules being "lazy", for example.
With #S_N2# reactions, a replacement occurs via backside attack, a spatial pathway that is fairly simple to figure out if the nucleophile is fairly strong (i.e. it has a strong desire to forgo its "laziness" and try harder) and has low steric hindrance (is not bulky).
With #E_2# reactions, the nucleophile has to be aligned antiperiplanar to the electrophilic center for the elimination to occur. That means the nucleophile has to move around until it finds the optimal position in space to do the elimination, which means it needs to use extra energy until it finds that perfect spot. If the nucleophile is fairly weak and is rather bulky, it convinces itself that it would rather not move around as much, and prefers to be "lazy". (Let's face it, who wants to spend more energy than they need to?)
If you raise the temperature, the nucleophile has more energy on average, and is much more OK with moving around until it finds the best spot for elimination because it's able to move much more quickly.
Now, let's look at a reaction coordinate diagram to see why an increase in temperature causes this, mathematically:
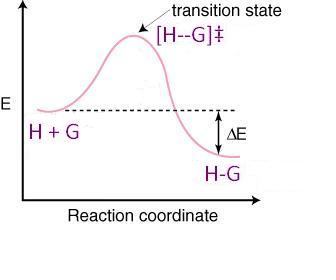
Recall the popular thermodynamics equation, modified for the transition state:
#DeltaG^‡ = DeltaH^‡ - TDeltaS^‡#
As temperature increases, #DeltaG^‡# gets less positive (or more negative) for the elimination reaction's transition state and that transition state becomes more and more favored. Thus, at higher temperatures, you get more elimination products than at lower temperatures.



