How does the ionic radius of a nonmetal compare with its atomic radius? Explain why the change in radius occur?
1 Answer
Jun 8, 2018
Well, non-metals are OXIDIZING....
Explanation:
And look at fluorine, and oxygen, these are potent oxidants...that accept electrons...
And thus the anions that result upon reduction...should be LARGER than the parent atoms...because electrons have been added to the valence shell...the electrons experience enhanced shieldng in the anion...
But as chemists, as physical scientists we should interrogate the data...
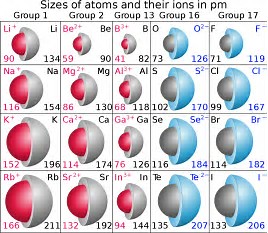
The units are in

