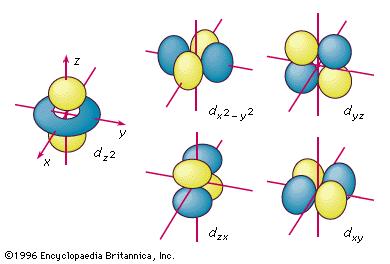
How many d orbitals can there be in one energy level?
1 Answer
Jul 20, 2016
This depends on the number of magnetic quantum numbers
#n# is the principal quantum number (the energy level) going as#1, 2, 3, . . . , N# , and#N# is a large integer. For one energy level,#n# is only one number at a time.#l# is the angular momentum quantum number, and it goes as#0,1,2, . . . , n-1# . For one subshell,#l# is only one number at a time, and#l = 0,1,2,3,4 . . . # corresponds to the#s,p,d,f,g, . . . # subshells. However, there can be more than one#l# for the same energy level.#m_l# is the magnetic quantum number, and takes on all numbers in the set#{-l, -l + 1, . . . , 0, . . . , +l - 1, +l}# . It corresponds to how many orbitals are in a subshell.
For an energy level to validly have
For any
#color(blue)(m_l = {-2,-1,0,+1,+2})#
So, there are
Example