How many moles of sodium nitrate, #NaNO_3#, would be produced from the complete reaction of 253g sodium chromate, #Na_2CrO_4#?
The reaction is #Pb(NO_3)_2+Na_2CrO_4 -> PbCrO_4 + 2NaNO_3# .
The reaction is
1 Answer
Jun 6, 2016
3.12 Moles of
Explanation:
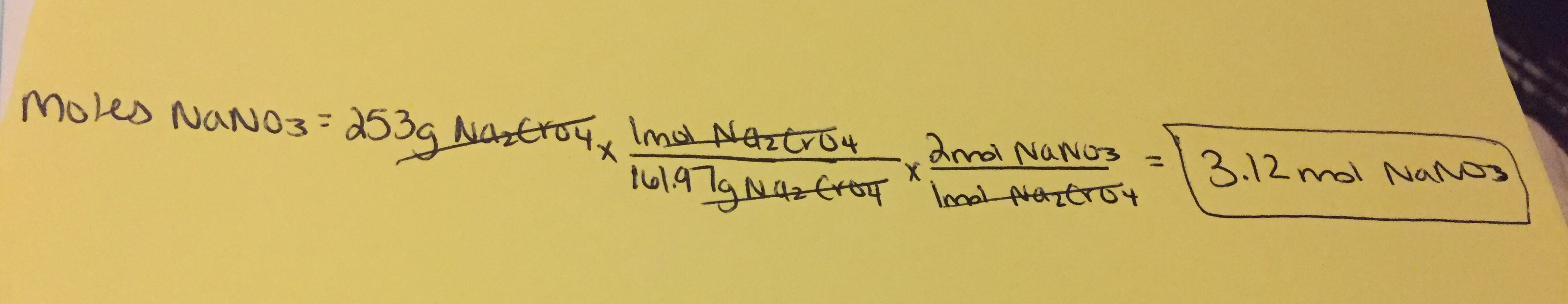
When performing mass to mole calculations, I use the following method:
Quantity Sought = Quantity Given x Conversion Factor
- The quantity sought is what we are trying to find, in our case we are trying to determine the number of moles of
#NaNO_3# - The quantity given is the value that we have in the problem, which is 253g of
#Na_2CrO_4# - The conversion factor is what allows us to go from grams of
#Na_2CrO_4# to moles of#Na_2CrO_4# , which ultimately leads us to the number of moles of#NaNO_3# .
The 161.97g represents the molar mass of
As you set up these type of problems the units that you no longer need should cancel out, leaving the unit you want as your end result.

