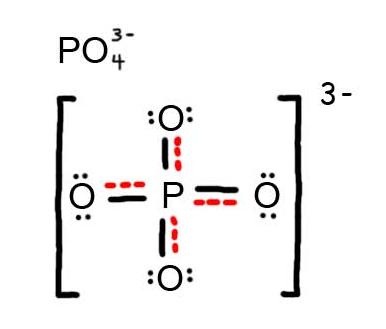
How many resonance structures can be drawn for #"PO"_4^(3-)#?
1 Answer
You can draw five resonance structures for
Explanation:
If you start by drawing four oxygen atoms single bonded to a phosphorus atom and give every atom an octet, you get Structure R in the diagram below.
This is not a "good" structure , because it has five atoms with formal charges.
We can reduce the number of charges by moving electrons to convert a
There are four
These are all equivalent and are the major contributors to the resonance hybrid.
Hence, the actual structure is a resonance hybrid of all five structures, with : S, T, U, and V being the major contributor and R a minor contributor.