How much 0.12 M #HNO_3# solution can be made by diluting 250 mL of 3.4 M #HNO_3#?
1 Answer
Jun 12, 2016
Explanation:
In order to get the answer, you have to use the following dilution equation:
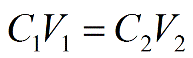
* TIP. * Whenever you are diluting a solution (going from a highly concentrated substance to a less concentrated substance by increasing the volume of the solvent) you always use this equation.
We know the initial concentration, inital volume, and final concentration. All we have to do rearrange the equation to solve for the final volume:

