How would you convert a ketone to an aldehyde?
1 Answer
Nov 13, 2015
One way you could do it is using trifluoroperoxyacetic acid (a Baeyer-Villiger oxidation), and then using diisobutylaluminium hydride (DIBAH) in Toluene in the presence of a dry ice bath. I'm unsure as to how these react in more complicated contexts, but let's take acetone and convert it to acetaldehyde:
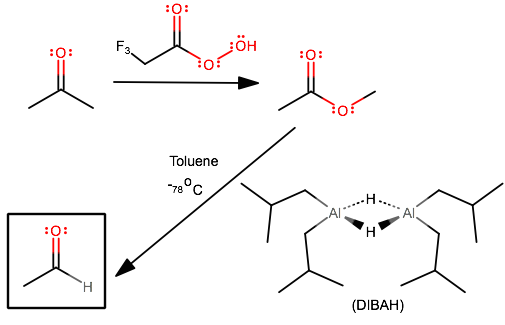
Neither mechanism is particularly well-known, but in general, in step 1, an oxygen is inserted in between a carbonyl and an adjacent carbon, and then for step 2, the

