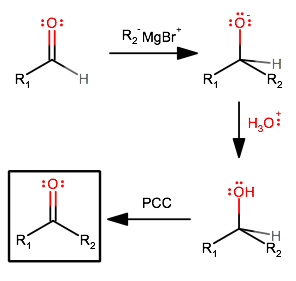
How would you convert an aldehyde to a ketone?
1 Answer
Nov 16, 2015
If you think about it, all you do is add a carbon, so what you need is a nucleophile that can attack at the carbonyl carbon that looks like an
A straightforward solution is
where
Overall:
- Attack the carbonyl with an alkyl Grignard reagent to tack on the alkyl group.
- The acid protonates the alkoxide to finish the first step, and the water that remains deactivates the alkyl Grignard reagent back into an alkane.
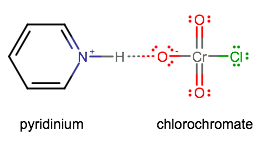
- PCC (pyridinium chlorochromate) oxidizes the hydroxyl group into a carbonyl group via a
beta -elimination of the explicit proton.
PCC looks like this:
What's special about it is that it oxidizes alcohols one step forward, i.e. primary alcohol to aldehyde, or secondary alcohol to ketone.