How would you draw an enthalpy diagram for: #N_(2(g)) + 3H_(2(g)) -> 2NH_(3(g)) DeltaH= -"100.3 kJ"#?
1 Answer
Here's how that would look like.
Explanation:
Start by having a look at the thermochemical equation the problem provides you with
#"N"_text(2(g]) + 3"H"_text(2(g]) -> 2"NH"_text(3(g]), " "DeltaH_text(rxn) = -"100.3 kJ"#
The important thing to notice here is that the enthalpy change of reaction,
What that means is that when nitrogen gas and hydrogen react to form ammonia, energy is being given off by the reaction to the surroundings
More specifically, when one mole of nitrogen gas reacts with three moles of hydrogen gas, two moles of ammonia are formed and
Now, a potential energy diagram is used to show how the energy level of the reactants changes during the course of the reaction, up until the formation of the products.
An exothermic reaction is characterized by the fact that energy in the form of heat is being given off to the surroundings.
Well, where is this energy coming from?
In this case, the energy level of the products will be lower than the energy level of the reactants. Simply put, this energy is coming from the reactants, because they were at a higher energy level when the reaction began.
So, here's how a rough sketch of a potential energy diagram would look like for this reaction
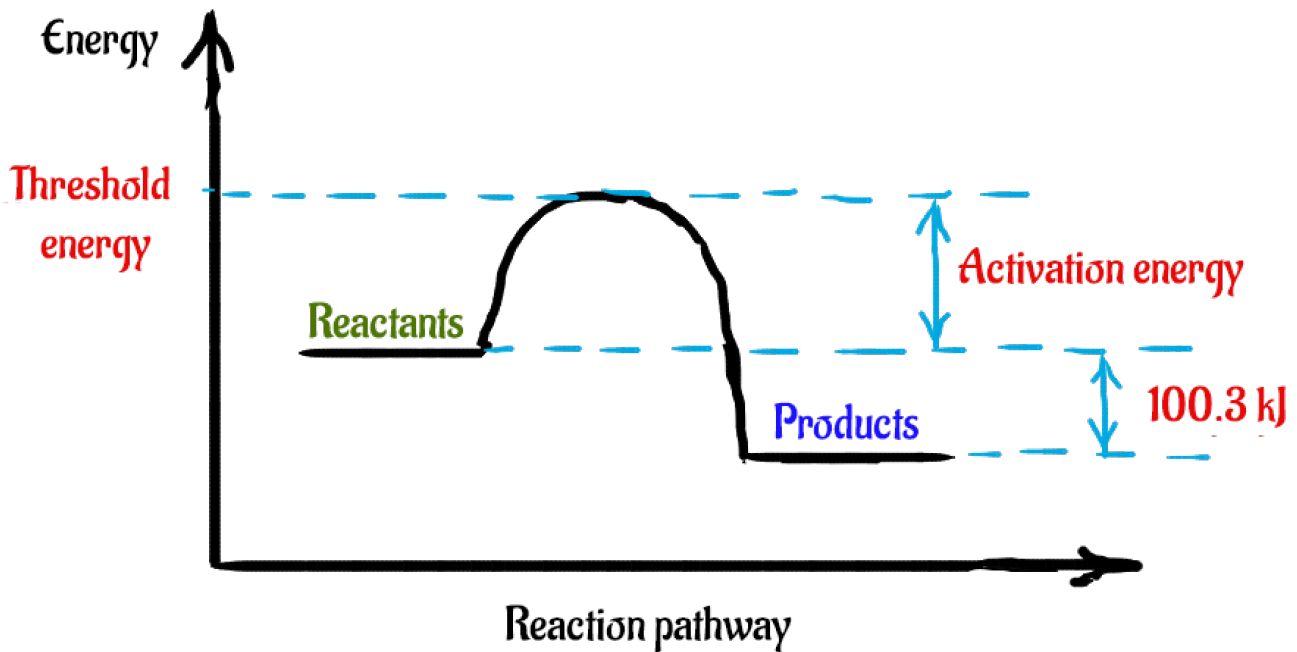
The difference between the energy level of the products and the energy level of the reactants is equal to the enthalpy change of reaction,
Since the products are lower in energy,
So, if you add enough energy to the reactants to get them to reach the threshold energy, the reaction will take place. This energy that must be added to the reactants is called activation energy.
More on threshold energy and activation energy here:

