How would you draw the skeletal structure of the product you would expect to result from the following reaction?
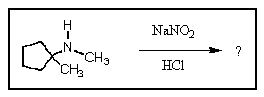
1 Answer
Mar 24, 2016
See below.
Explanation:
This is a reaction of nitrous acid with a secondary amine.
Nitrous acid is unstable and is always prepared immediately before use by reacting sodium nitrite in the cold with a strong acid like
Nitrous acid is a weak acid, so you get the reaction:
The nitrous acid reacts with a secondary amine to form a nitrosamine:
Your compound is a secondary amine. The structure of the product is therefore
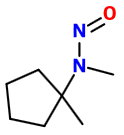
Nitrosamines are insoluble yellow oils.
They are powerful carcinogens — Be extremely careful when handling them!

