How would you draw tryptophan and select the chiral carbon?
1 Answer
Nov 24, 2015
The chiral carbon, if there is one, tends to be the
Tryptophan is the only amino acid that has two rings on it. At pH 7.4, it looks like this:
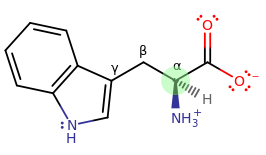
You can see the chiral carbon highlighted. You can tell that it's an

