Hydrogen bonding is of two types - t Intramolecular and intermolecular Am I right ?
1 Answer
Nope. It is only intermolecular.
It is just a misnomer that we call it "hydrogen-bonding". Yes, the hydrogen is bonded within the molecule, but the term "hydrogen-bonding" does not refer to that.
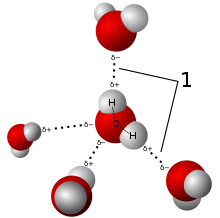
It refers to the polarizing interaction with an electronegative atom on another molecule, which strictly speaking, is a significantly weaker interaction than in a regular bond.
This interaction is...
the polarization of electron density from an adjacent molecule's
#H# atom by an electronegative-enough atom.
Often we speak of
Thus, there is particularly noticeable hydrogen-bonding in
It is not, however, restricted to

