If a compound has a chiral center, is it chiral?
1 Answer
The answer can be yes and no.
Explanation:
A molecule has a chiral centre (non-superimposable mirror image) when four different substituents are attached to an atom in a molecule, for example:

Image from:
In the molecule above the chiral centre as shown (fourth different atom is a Hydrogen) makes the molecule as a whole a chiral molecule, in other words it would rotate polarized light.
A molecule is not chiral, known as achiral, when it has (a) chiral centre(s), but the molecule on the whole is symmetrical (another name for this is a meso compound). An example of a meso compound is shown below:
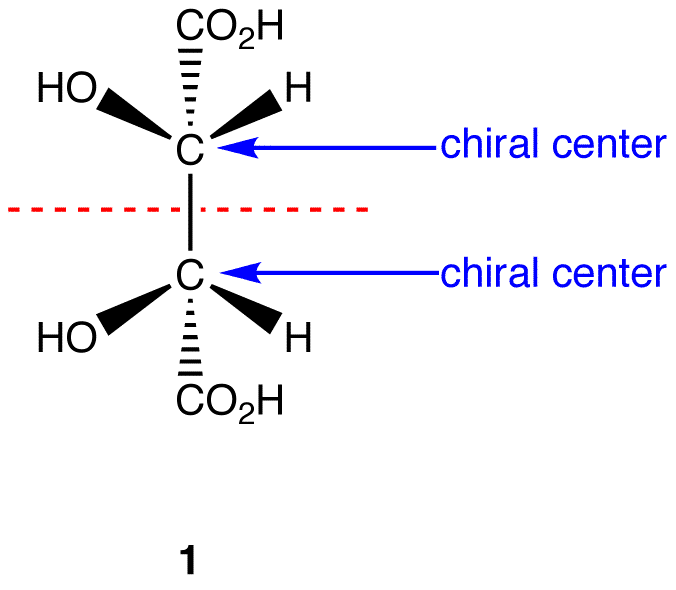
Image from: http://chemwiki.ucdavis.edu/Organic_Chemistry/Chirality/Meso_Compounds
As can be seen by the red dotted line, the molecule has a plane of symmetry and even though it has two chiral centres, the symmetry makes the molecule achiral and thus it would not rotate polarized light.
Hope I helped :)

