If an element's electron configuration ends in #ns^2 np^5#, what is the element?
2 Answers
The element is member of the halogen group.
Explanation:
The halogens are group 17 on the periodic table and include F, Cl, Br, I
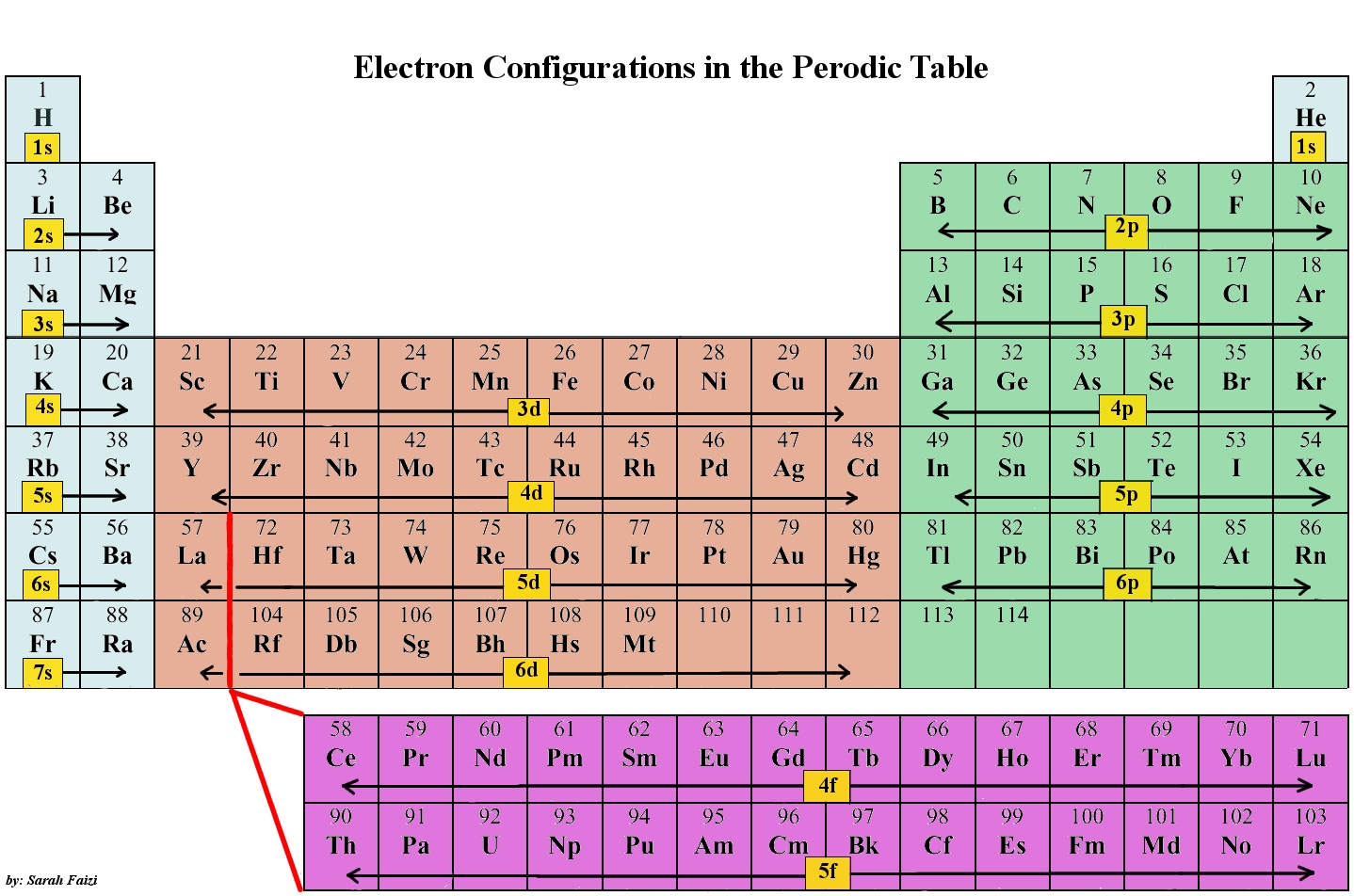
As you can see, the
So, if you were to find what elements have 5 electrons in the
Hope that helped :)