If given this balanced equation: #2C_7H_6O + C_3H_6O -> C_17H_14O +2H_2O# Why is the acetone deprotonated and not the benzaldehyde?
1 Answer
May 27, 2016
As explained below
Explanation:
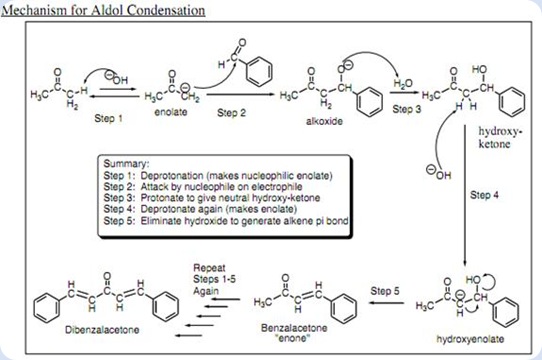
The reaction occurs according to the mechanism as shown above via the formation of carboanion (
The deprotonation of acetone is thus favored because this stabilization of deprotonated ion from bezaldehyde is not possible.
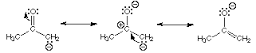
The resonance structures of the anion obtained by deprotonation of acetone

