If pyridine and pyrimidine are both aromatic compounds, how many elctrons does each nirtogen atom contribute to the electron cloud of each compound?
1 Answer
Sep 13, 2016
In each compound, each
Explanation:
Pyridine
The structure of pyridine is
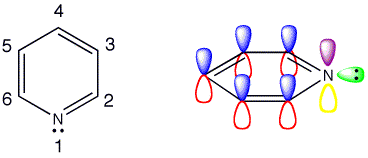 www.chemtube3d.com
www.chemtube3d.com
That leaves 1 valence electron available to participate in the aromatic π cloud.
Pyrimidine
The structure of pyridine is
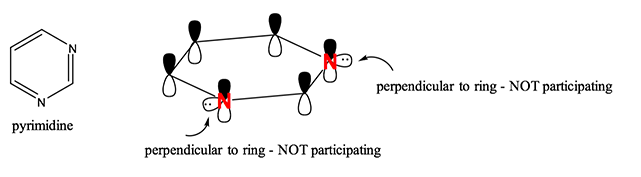 www.studyorgo.com
www.studyorgo.com
The two
Each
That leaves 1 valence electron available in each

