If you knew the number of valence electrons in a nonmetal atom, how would you determine the valence of the element?
2 Answers
The possible valances can be determined by electron configuration and electron negativity
Explanation:
All atoms strive for stability. The optima electron configuration is the electron configuration of the VIII A family or inert gases.
Look at the electron configuration of the nonmetal and how many more electrons the nonmetal needs to achieve the stable electron configuration of the inert gases. Non metals tend to be negative in nature and gain electrons. ( They are oxidizing agents)
For example Florine atomic number 9 needs one more electron to reach a valance number of 8 electrons to equal Neon atomic number 10. Hence Flowrine has a valance of -1
Oxygen atomic number 8 needs two more electrons to reach a valance number of 8 electrons to equal Neon atomic number 10. Hence Oxygen has a valance charge of -2.
Non metals with a low electron negativity will lose electrons when reacting with another non metal that has a higher electron negativity. When the non metal forms an ion it is necessary to look at the electron structure to determine how many electrons the element can lose to gain stability.
For example Chlorine which is normally -1 like Florine when it combines with oxygen can be +1, +3, + 5 or +7. It can lose its one unpaired electron, or combinations of the unpaired electron and sets of the three pairs of electrons.
You would subtract the number of valence electrons from eight.
Explanation:
The valence of an atom is as the number of hydrogen atoms that it can combine with.
Since a hydrogen atom can form only one bond, valence is also the number of bonds that it can form.
Four valence electrons
Carbon has four valence electrons.
It can form four bonds.

Five valence electrons
Nitrogen has five valence electrons.
It can form three bonds.
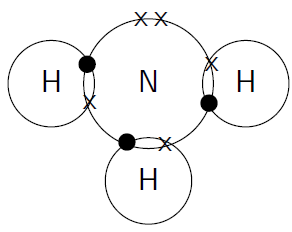
(Adapted from Boundless)
Six valence electrons
Oxygen has six valence electrons.
It can form two bonds.
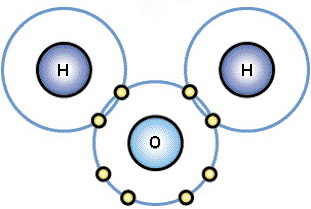
(Adapted from Biology @ IUPUI)
Seven valence electrons
Chlorine has seven valence electrons.
It can form one bond.

Make a Rule
Do you see a pattern? Can you make a rule?
The valency
#color(blue)(bar(ul(|color(white)(a/a)V + VE = 8color(white)(a/a)|)))" "#
or
#color(blue)(bar(ul(|color(white)(a/a)V = 8 - VEcolor(white)(a/a)|)))" "#

