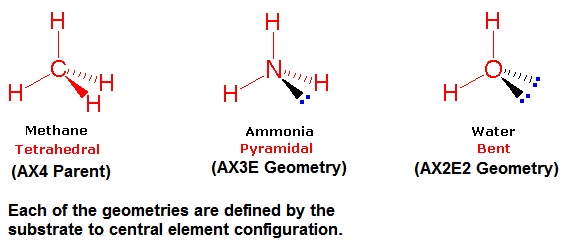
In the Lewis dot structure for #NH_3#, the central atom of the molecule has three atoms bonded to it and one lone pair of electrons. What is the shape of an #NH_3# molecule?
2 Answers
Trigonal pyramidal
Explanation:
An ammonia molecule has nitrogen as the central atom with three hydrogen bonds and one lone electron pair. This gives the molecule a steric number of four. Therefore, the molecular shape is trigonal pyramidal.
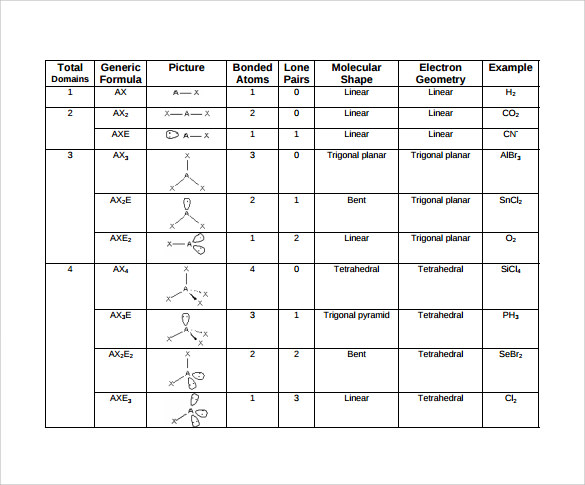
Pyrimidal
Explanation:
A method for determining geometry of binary compounds.
Given a binary compound
Needed is the number of 'Bonded electron pairs' (BPrs) and the number of 'Nonbonded electron pairs' (NBPrs). The total number of bonded pairs and nonbonded pairs => the Parent Structure. The Geometry is defined by the 'bonded pairs' attached to the central element of the Parent Structure. The nonbonded pairs occupy the remaining orbitals of the parent structure but are not used to define the final geometry. The table posted in the 1st answer illustrates this concept.
To Determine the Geometry of a Binary Compound ...
Determine the Number of Bonded Pairs
=> number of Substrate elements attached to the central element.
#NH_3# has 3 bonded electron pairs b/c there are 3 Hydrogens bonded to the central element, Nitrogen.
#BPrs = 3#
Determine the Number of Nonbonded Pairs (
a. Determine the 'Valence Number'
=>
#V_e# = Valence electrons of A + n(Valence electrons of X)
#"For"# #NH_3# =>#V_e# =#3H + 1N# =#3(1) + 1(5)# =#8#
b. Determine the 'Substrate Number'
=>
#"For" NH_3# =>#S_e# =#3H = 3(2) = 6#
c. Determine the number of non-bonded electron pairs.
#NBPrs = ((V_e - S_e)/2) = E_n#
#"For"NH_3# =>#NBPrs = ((8 - 6)/2) = 2/2 = 1 NBPr#
Determine Parent Structure:
Determine Geometry of Structure of Interest: