Is covalent bond formation endothermic?
1 Answer
Aug 3, 2014
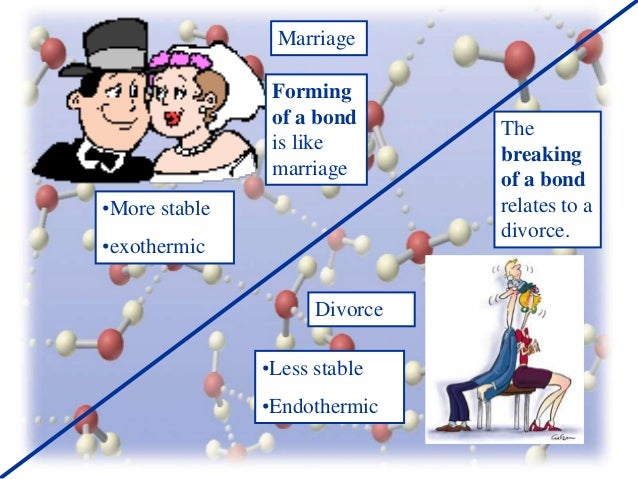
No. It is exothermic.
Covalent and any other kind of bonds owe their stability to the fact that the total energy of the bonded atoms is lower than the sum of energies of the unbounded atoms.
The excess energy is released, thus determining the exothermic character of bond formation.
If the formation of a bond were accompanied by an increase of energy,the bond just wouldn't form at all, as in the case of two atoms of helium.
I hope this will solicit more questions.