Is the amount of #Na(OH)# with respect to amide in hydrolysis important?
1 Answer
Yes. With acid-catalyzed hydrolysis towards the carboxylic acid, it is generally easier, but with base-catalyzed hydrolysis towards the carboxylate, it requires that
Amides are not the most reactive carbonyl species (thank god, or else we'd turn to mush; how about them peptide bonds?), so using
In accordance with Le Chatelier's principle, the more
The mechanism is:
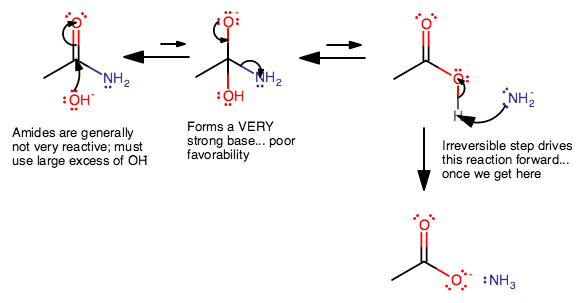
The first step, as we mentioned, is difficult, so we must add excess
The second step is also difficult; forming
Once we get past that,

