Is the peptide bond easily broken? Why would this be important?
1 Answer
No, of course not.
All major proteins in our bodies contain peptide bonds that hold together their backbone.
A peptide bond is:
For example, in an alpha helix, we have plenty of peptide bonds in the amino acid fragments connecting across each rectangle in this diagram:
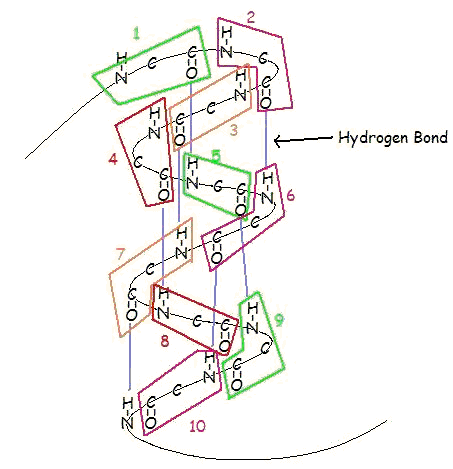
And in a beta-pleated sheet, we also have plenty of peptide bonds:
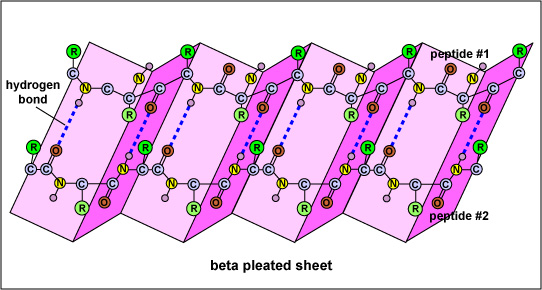
Both of these structures are found in many proteins.
If peptide bonds were hydrolyzed quickly and easily, then we would probably not be alive (no kidding).
Actually, in the first one or two weeks in biochemistry I was taught that peptide bond hydrolysis is thermodynamically favorable but kinetically unfavorable (half-life: about 1000 years).
In the amount of time it would take to hydrolyze a peptide bond, another one would probably be formed in its place.

